|
ramé-hart instrument co. October 2015 Newsletter |
| Is Your Superhydrophobic Surface the Real Deal...Or Is It a Fake? And What is Pseudosuperhydrophobicity Anyway? |
|
Over a dozen times in the past ten years, the focus of this newsletter has been on superhydrophobic surfaces - how to make them, how to measure them, why we need them, and how to improve them.1 But why so much attention? Superhydrophobicity represents the most exciting question in surface science today. Novel methods like laser-etching, lithography, deposition, and sol-gel are being used and developed to fabricate in larger quantities and at lower costs durable surfaces on commercial products - from windshields to toilet plungers - that improve self-cleaning properties, reduce maintenance costs, and elevate the world's quality of life in myriad ways. Researchers Dettre and Johnson discovered in the 1960s that superhydrophobic surfaces found in nature, such as the Lotus leaf, are a function of hydrophobic materials and roughness. They developed models to explain this novel phenomenon. In the 1970s Barthlott and Ehler developed models for self-cleaning superhydrophobic surfaces using micro-nanostructured surfaces. During the 1980s and into the 1990s a wide spectrum of methods and models emerged to explain and develop superhydrophobic surfaces. In 1997 Barthlott and Neinhuis described the Lotus effect. Methods for producing durable superhydrophobic surfaces with hierarchical structure were introduced shortly thereafter. In recent years, many new methods have been and are being developed to produce lotus-like and other superhydrophobic surfaces. The Lotus leaf is the prototypical forbearer of any hierarchically-structured superhydrophobic surface that exhibits the Cassie wetting regime. This type of surface features a self-cleaning property, low roll-off angle, and very low contact angle hysteresis. This behavior can be explained by the air pockets under the drop that keep the liquid in contact only with the asperities of the surface - thus giving it very little surface contact with which to hold on. This type of superhydrophobic surface is the real deal, the genuine article, the real McCoy.
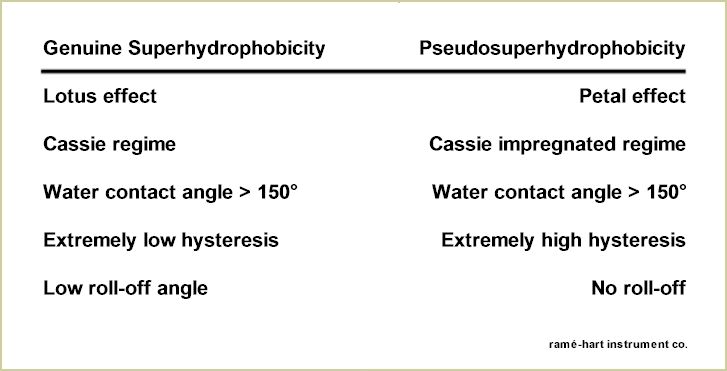
Let us be careful, however, not to simply identify any surface with a water contact angle in excess of 150° as superhydrophobic. While a genuine superhydrophobic surface will also exhibit low hysteresis and low roll-off angle, there are other surfaces that exhibit extremely high contact angle but also high hysteresis and no propensity to roll off. Bormashenko and others refer to this behavior as pseudosuperhydrophobicity.2,3 In other words, fakes. The "rose petal effect" is used to identify these pseudosuperhydrophobic surfaces. On a rose petal, micropapillae populate the surface. Each papilla has a series of nanofolds. The hydrophobicity of the surface is enhanced by a hierarchal structure of micro- and nanoscopic topology. However, the wetting behavior is starkly different. In the case of a genuine superhydrophobic surface which by nature is in a Cassie regime, a water droplet will sit above both the micro and nanostructures as shown in the diagram below on the right. With the petal effect, the drop will rest in a Cassie impregnating wetting regime as shown below on the left. With the drop embedded into the nooks and crannies of the larger microscale topology of the surface, the propensity to wet and dewet are very low which explains the sticky behavior and why a water droplet will stay affixed to a rose petal even when it's turned completely upside down. The much-studied Gecko feet work on the same principle as the rose petal - both display high contact angle and high adhesion. The table below details the differences between pseudosuperhydrophobicity and the genuine article. Now, the next time someone wants to sell you on their superhydrophobic surface, you have the tools to determine if it's the real deal...or a counterfeit. Let this also serve as an example of why contact angle alone cannot always give us the full picture of wetting behavior. By adding a roll-off or advancing and receding contact angle measurement, we can quickly identify if a superhydrophobic surface exhibits the Lotus effect or the Petal effect - even if the high contact angle is the same between them.
Lastly, we should point out that while both the Cassie and Wenzel regimes are sufficient for explaining wetting behavior on surfaces with one scale of topology, they become less helpful with describing wetting behavior on surfaces with topology at multiple scales (e.g., nano and micro). Furthermore, while surfaces with a hierarchal structure may be superhydrophobic, only those with low hysteresis can be said to model the Lotus effect. The spacing and height of the microstructures determine whether a Cassie or Cassie impregnated wetting regime will result. While the microstructure determines if wetting behavior will follow the Lotus or Petal effect, the nanostructure drives the high contact angle in both cases. 1 See
http://www.ramehart.com/newsletters.htm. |
| Ten Years Old |
|
This newsletter, in its present form and
written by the undersigned, just had its tenth birthday. Published
monthly twelve times per year, we have not missed a single issue. Each
month we strive to find some noteworthy aspect of surface science and
specifically related to contact angle and wetting to share with you.
Some months we receive valuable feedback and kind words of praise from
our readers. Other months we receive corrections which we also
appreciate.
If you have any feedback regarding this
or any of our newsletters, or if you have an interest in any of our
products, please feel free to
contact
us today. |
|
Regards,
Carl Clegg |