|
March
2017 |
|
| The Effect of Drop Volume on Contact Angle | |
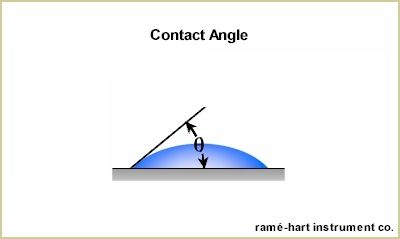
| When measuring static contact
angle, a liquid drop is deposited on the solid surface forming a
sessile drop. The drop will typically have a volume between 1µl and
8µl. One of the most commonly asked questions is, "What is the
correct drop volume for measuring static contact angle?" While there
is not a single correct answer, there are some considerations that
are worth exploring.
If the drop is too large - say over 10 to 20µl or larger - the effects of gravity pull the drop down and distort the contact angle. If the drop is too small - say under 1µl - not only is it more difficult to dispense and view, but smaller drops will also evaporate much faster, lack axisymmetry, and often exhibit a high standard deviation among a series of measurements on the same surface for reasons explained below. An ideal solid surface is one that is smooth (i.e., no roughness), rigid, chemically homogeneous, insoluble, and nonreactive.1 Likewise, an ideal contact angle is the static, or equilibrium contact angle on an ideal surface. In the case of an ideal surface, it doesn't matter what the drop volume is. Whether it's 1µl or 5µl, the resulting static contact angle should be the same. In the real world, it turns out that most surfaces are not ideal. This means that drop volume can matter. Generally, as the contact angle hysteresis2 increases, the less ideal the surface is. In other words, an increase in contact angle hysteresis indicates an increase in roughness, a decrease in chemical homogeneity, or some combination of the two. Contact angle hysteresis is the difference between the maximum possible contact angle, referred to as the advancing contact angle, and the minimum, or receding contact angle. One method for measuring advancing and receding contact angle is the add/remove volume method illustrated in the graphic below.3
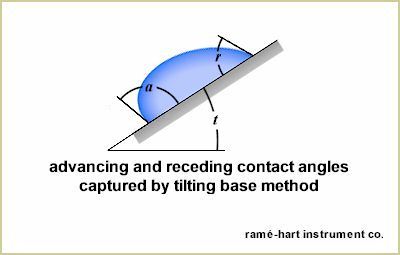
Another method, illustrated below, is the tilting base method. If the drop rolls off at a low tilt (t) angle, the contact angle hysteresis is generally low. It's important when using the tilting plate method that uniform drop volumes are used. On a given surface, as the drop volume is increased, the tilt angle at roll off decreases due to the effects of gravity (except in cases when Wenzel state is present).
Since most surfaces exhibit some contact angle hysteresis and thus are less than ideal, it's recommended that the same drop volume be used when testing multiple locations on the same sample or multiple variants of the material. Additionally, hydrophobic surfaces will need larger drop volumes than hydrophilic surfaces. Bullets
Notes 1 Abraham Marmur,
"Solid-Surface Characterization by Wetting", Annu. Rev. Mater. Res.
2009. 39:475. Keywords Drop volume,
ideal surface, ideal contact angle, contact angle, contact angle
hysteresis, advancing contact angle, receding contact angle, surface
roughness, surface heterogeneity, tilting plate method, add/remove
volume method, standard deviation, drop axisymmetry |
|
| Product of the Month: Model 290 | |
|
Eight years ago we introduced Model 290.1 This incredibly versatile model has become one of our top-selling products and is well-suited for all types of contact angle measurements, static and dynamic, advancing and receding, as well as surface tension, interfacial tension, and surface energy studies, and much more. Model 290 includes our powerful DROPimage Advanced software, Automated Dispensing System, and Automated Tilting Base.
If your lab could benefit from a powerful and multipurpose surface science tool, please contact us today for more information and a quotation for our Model 290. 1 See our July 2009 Newsletter. |
|
|
Regards,
Carl Clegg |
|