|
June 2019 |
| Ten Reasons Why Water is Weird |
|
Apparently there are a lot of people out there who think water is weird. After looking into their reasons, I have joined their ranks. Today, I share with you just ten of the reasons why "Water is Weird". 1. Unlike almost every other liquid, water expands when frozen. Were this not so, ice would sink to the bottom of lakes and oceans, freeze solid, and marine life as we know it today would cease to exist. Since ice does float, it can insulate the water under the surface and enable living things to not just survive but thrive. 2. You may think that water can exist in three different states: steam, liquid, and ice. And yes, while that's true, there's much more to the story. In fact, researchers have discovered at least 18 different phases of ice - if you can believe that.1 One of the phases can grow 1000 miles per hour.2 Under the right conditions, the entire world could freeze in a matter of hours. Fortunately, that hasn't happened...yet. In fact, if you're careful, you can supercool water - that is, bring its temperature down below its freezing point without crystallization occurring.3 3. Hot water freezes faster than cold water. Who would have thought? Back in the 1960s, a high school student in Tanzania discovered a hot ice cream mix froze faster than a cold mix did. When the behavior was confirmed by a visiting physicist, it was named the Mpemba Effect, after the student who discovered it. Fifty years later, scientists are still struggling to explain this odd behavior.4 4. Water is amazingly difficult to compress. Ocean water, even at 2 miles below sea level, compresses less than 1% by volume despite being at a pressure of over 4700 psi. The lack of compressibility is actually a good thing. It makes it easier to move around. Think of water pistols or garden hoses. 5. It's hard to find water in a pure state - almost everything sticks to it - which can be a good thing. The blood coursing through your veins is really mostly water with some salts and proteins floating around or dissolved in it. In fact, a lot of things can dissolve in water making it one of the most corrosive and reactive chemicals around. 6. Water may be tasteless, colorless, and contain no calories, but without it, there would be no life. In fact, it's the second most common molecule in the universe.5 7. Aside from mercury (which is also weird), water has about the highest surface tension of any known pure liquid. Water molecules are pulled equally in all directions by cohesive forces. See the blue area in the graphic below. However, on the surface (see green area), there are fewer molecules and so the cohesive forces are stronger pulling the molecules inward.
A drop of water without gravity would take the shape of a sphere. And the outside surface would behave like a thin rubber sheet holding all the molecules together. Since we do have gravity here on earth, the drop is pulled down toward the earth until it hits other matter. If the matter it rests on is hydrophilic, the drop will spread out and exhibit a low contact angle. This is the result of a strong adhesive force between the drop and the surface. In this condition, wetting is said to be good. If the matter is hydrophobic, then wetting is poor, the cohesive forces on the surface of the drop are strong than the adhesive forces between the drop and the solid and the drop will bead up more like a ball and the contact angle will be high. We are especially interested in wetting because we build tools for measuring surface tension as well as the contact angle. It's high surface tension that allows insects and paper clips to float on water. 8. It's also surface tension that drives capillary action - allowing water to run uphill. That's also a good thing. Without capillary forces, plants wouldn't get the water they need and your brain would dehydrate. 9. The Leidenfrost Effect explains how a drop of water can dance around on a hot surface without evaporating. What's really happening is a layer of water on the bottom of the drop vaporizes due the heat but then, not able to escape, suspends the drop above the surface insulating it from the heat. With careful tuning, drops can even be made to climb uphill.6 10. You may have heard that no two snowflakes are alike.772 That may be true but hard to prove. It's said that even the most minute changes in temperature and humidity can alter the growth of a snowflake. Even though we only have space for ten reasons why water is weird, no doubt there are many more reasons. We like water because it's the most efficient liquid for measuring contact angle. It's cheap, easy to use, and has a high surface tension which lends itself to characterizing wetting properties. Maybe in another edition of this newsletter, we'll explain why we use deionized water. Until then, keep being weird. Notes |
| A Variety of Wetting Behavior |
|
Wetting is a balance between adhesive
and cohesive forces as referenced in point 7 in the above article.
With many surfaces, a sessile drop deposited on the surface will
remain there statically. It's easy to measure the static contact
angle of such a drop on such a surface since not too much is going
on except perhaps a little evaporation which happens relatively
slowly. This is due to the fact that on most surfaces the pinning
effect is stronger than any change in adhesive or cohesive forces.
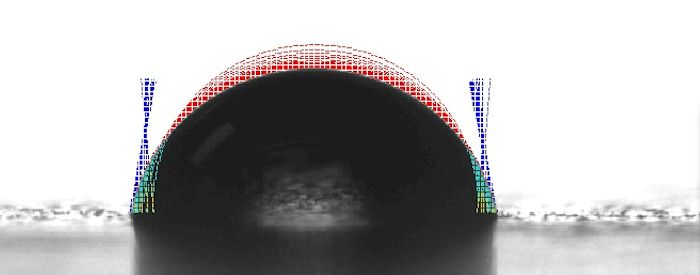
Nonetheless, there are some surfaces on which dynamic wetting will occur. On Surface A shown in the graphic below, the contact angle is decreasing over time. Simultaneously, the drop width is increasing, the drop height is decreasing, and the drop volume is remaining relatively static.
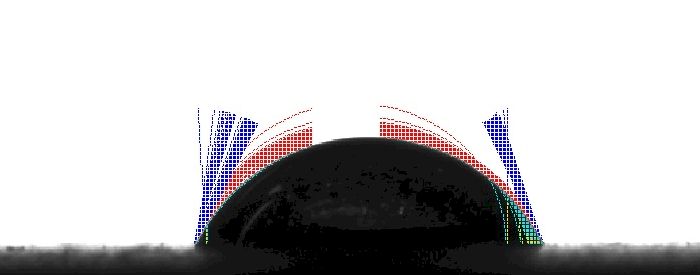
There are a number of possible explanations for this behavior. For example, if the surface is treated with a chemical which acts as a surfactant, the surface tension of the drop decreases as the chemical interacts with the water; the cohesive forces on the outer molecules of the drop weaken and the drop spreads (i.e., wets). In the case of Surface A above, the wetting is uniform which indicates that the surface is homogenous in terms of chemical composition. In the case of Surface B below, the left side is pinned while the right side is moving or wetting out. This would indicate that Surface B is not as homogenous with respect to chemical composition or surface roughness.
Sometimes the contact angle will drop quickly and the width may even increase as the drop decreases as shown on Surface C below. However, since the drop volume is also dropping quickly (also observable in the experiment report), the behavior is not so much wetting but absorption. The drop is being absorbed into the mildly porous surface.
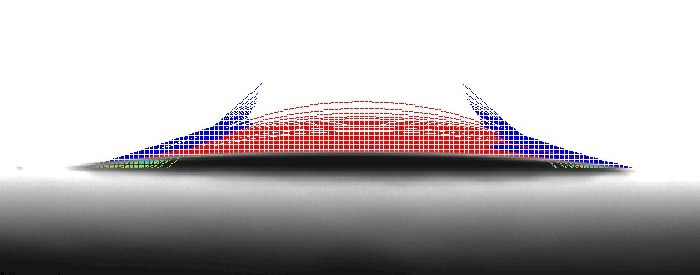
In the case of Surface D below, the contact angle of the left side is increasing as the contact angle of the right side decreases. As we are taking measurements, we are also tilting the sample. The final left measurement (on the downhill side) is the advancing contact angle. The final right contact angle (on the uphill side) is the receding contact angle. The difference between these two contact angles is referred to as the contact angle hysteresis. When the contact angle hysteresis is relatively small, the surface is relatively homogenous with regard to both chemical as well as structural composition.
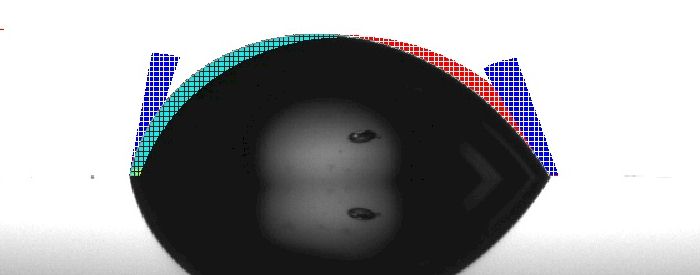
Surface E introduces an interesting and less common phenomenon. The drop actually started as a solid. The surface is heated to a temperature greater than the melting point of the drop material. Once the drop begins to form, we measure the contact angle. As the drop forms, you will see that the contact angle decreases while the drop width increases. In general, as temperature increases, surface tension decreases. As surface tension decreases, wetting improves because the cohesive forces on the surface of the drop are weakened relative to the adhesive forces between the drop and the surface.
In short, there are a number of reasons why drops have a hard time sitting still. If you have ever observed young children, you will note that they too often exhibit the same behavior. |
|
Regards,
Carl Clegg |