|
September 2020 |
| The Quest for an Ideal Surface |
|
I spent the first half of my life on a
quest for the ideal woman. I'm pleased to report that the endeavor was a
complete success. Shortly, we will be celebrating 30 years of marriage. And
as of late last week, we are now first time grandparents. How could
life get any better?
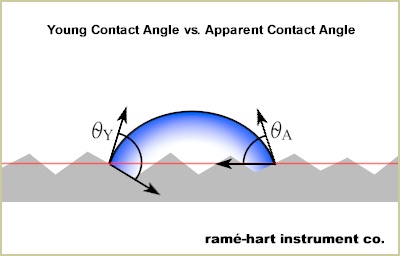
Now I've spent the second half of my life on a quest for an ideal surface. But sadly I report that I have not yet found one. Let's face it, the ideal surface is the Holy Grail of surface science. Many search but no one finds. This month, let's explore what exactly defines an ideal surface. But first, a quick review of Young's equation. The graphic below details the three forces that explain the contact angle of a liquid drop on a solid surface. This is the most basic and common mathematical description of wetting behavior. However, in order to use Young's equation to understand wetting of a liquid sessile drop in equilibrium on a solid surface, it's assumed that the solid is an ideal surface. However, most if not all surfaces are not ideal. We call these non-ideal surfaces real surfaces. Here are the characteristics of the ephemeral ideal surface:
It should be noted that an ideal surface will have no contact angle hysteresis. (This point appears to be hotly debated but we'll save that discussion for a future newsletter.) We observe that real surfaces have some contact angle hysteresis and we conclude that the magnitude of the hysteresis is a reflection of the noncompliance to the rules of ideal surfaces as listed above. When measuring contact angle on real surfaces, it's important to differentiate between the Young contact angle (θY) and the apparent contact angle (θA). The difference can be significant. When measuring a static contact angle using a goniometer, it's the apparent contact angle that's being measured. The angle is defined tangent to the drop and the surface baseline (red) as shown in the graphic above. The Young contact angle is measured between the drop tangent and the surface tangent at the microscopic level as illustrated above. With an ideal surface, the Young contact angle (θY) and the apparent contact angle (θA) are the same and cannot exceed about 120°. PTFE is an example of a material that approaches that maximal value when smooth and unstructured - that is, close to ideal. When an apparent contact angle of any material is measured in excess of 120°, it's not the underlying material alone that promotes the superhydrophobic behavior - rather, it's the surface roughness or topography. Surface roughness amplifies wetting behavior. That is say that roughness result in better wetting for hydrophilic surfaces and less wetting for hydrophobic surfaces. Low contact angles get lower and high contact angles get higher. When the contact angle hysteresis is low, it signals that the surface is close to ideal - or, in other words, the surface has little roughness and limited heterogeneity. The exception to this rule are surfaces in the Cassie-Baxter state - they nearly always have low contact angle hysteresis but high levels of roughness. So, if in your travels you find an ideal surface, be sure to measure the contact angle hysteresis. If it's near zero, you may have a winner. And if you can't find an ideal surface, you may want to start looking for an ideal mate. That will be you more joy in the long run anyway. Bullets
|
| Glossary |
|
We have had a glossary of surface
science terms on our website for many years. Recently, we updated
it. If you haven't visited it, take a look
here.
If you would like to suggest an addition, please
contact us with your suggestion. We always appreciate your
feedback. |
| Coupon Savings |
Interested in learning more about contact
angle? Check out this free book offer. Go to this
page, add to cart and follow the instructions in the coupon below to
save $50. Minimum order applies.
 |
|
Regards,
Carl Clegg |