|
March 2021 |
| Seven Factors that Drive Wetting Behavior |
|
Consider this month's newsletter
article to be a review. That is, we are not going to present any new
theories, materials, discoveries, or ideas that we haven't already
talked about in past newsletters. Rather, we're going to
repackage some key points that we've discussed discretely in the past as we
attempt to explain what variables actually affect wetting behavior.
Just to make sure we start out on the same page, wetting is commonly measured by observing the behavior of a liquid drop on a surface using optical goniometry. If the drop spreads out, wetting is said to be good, and contact angle is low. Conversely, poor wetting results in high contact angle or a hydrophobic condition. In this article, we will review seven factors that affect wetting behavior: Surface Energy. While it may be obvious, a solid's surface free energy can greatly affect wetting behavior. Polymers with low surface energy, such as PTFE, are difficult to bond or print or paint on. PTFE has a surface energy under 20 mN/m while most bonding applications require over a surface energy greater than 40 mN/m. As surface energy increases, wetting improves. Generally, cleaning processes and surface modifications seek to increase surface energy which, in turn, improves adhesiveness.
Surface Tension. The surface tension of the liquid affects its wetting property. Water, for example, has a relatively high surface tension. According to Young's equation, one of the forces that explains the contact angle is the liquid surface free energy. High surface tension liquids have greater molecular cohesive forces which cause drops to bead up. If you place a drop of water and a drop of oil on most any surface, you will see that the water contact angle will almost always be greater than the oil contact angle. Assuming the solid surface energy is the same for both drops, the only difference then is the liquid surface free energy which is higher in water. If a surfactant is added to the liquid phase, the surface tension will decrease and wetting will increase.
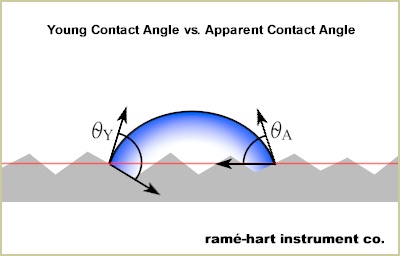
Roughness. When contact angle is measured on any surface that exhibits roughness (i.e., virtually all real surfaces), we're actually measuring the "apparent contact angle" as illustrated below. However, the actual or "Young contact angle" is greater. On hydrophilic surfaces (i.e., when the apparent contact angle is below 90°), there is an inverse relationship between contact angle and roughness. That is, as roughness increases, contact angle decreases. On hydrophobic surfaces, the opposite behavior is observed: as roughness increases, so too does contact angle.
Contamination. A fouled surface will retard wetting. Contaminants on the surface sit between the drop and the surface and weaken the strength of adhesion. A clean surface provides optimal wetting and contact angle will be lower. In adhesion applications, a clean surface is always preferred. During the fabrication of silicon wafers used to make semiconductors, for example, contact angle is used to test surface energy and relative cleanliness as even very small traces of contaminants can result in poor bonding with the photoresist which translates to increased rejections rates.
Surface Modification. Corona treatment, laser radiation, and plasma activation are examples of surface modifications. These treatments change the surface energy of the solid surface by depositing different functional groups on the surface. Corona treatment, for example, oxidizes the surface and increases the surface energy by depositing polar groups on the surface. The improved wetting behavior prepares the surface for subsequent processes such as coating, bonding, and painting. But be aware that that many surface modifications fade with time. Environmental Factors. Then there are environmental factors. As temperature increases, the intermolecular forces in the liquid phase become more active and the surface tension decreases which results in greater wetting, or lower contact angle. Humidity on the other hand tends to inhibit wetting; in most cases, as humidity increases, so too does the contact angle. Surface Topology. Asperities and engineered nanoscopic topologies can induce a condition referred to as the Cassie State - that is, the sessile drop sits on top of the asperities as shown below resulting in very high contact angles. This superhydrophobic condition is not possible on flat surfaces. Superhydrophobic surfaces are found in nature. The Lotus leaf is famously referenced; water contact angle on Lotus leaves are typically in the neighborhood of 160°. By contrast, when the drop sits fully on the surface - including in all the nooks and crannies, it's said to be in a Wenzel state. Different mathematical modeling methods are used to explain these two different states.
Chemical Homogeneity. When a non-nanostructered surface (i.e., one that demonstrates the Wenzel state) produces a small contact angle hysteresis (the delta between the advancing and receding contact angles), the surface is chemically homogenous. When multiple contact angle measurements at various locations on a surface exhibit a low standard deviation, this too would point toward a high degree of chemical and structural homogeneity. Can you think of any additional variables that affect wetting? If so, please email me and let us know. We may even choose to mention them in a future newsletter. Some researchers
question whether contact angle measurement is even necessary in
order to determine the wetting properties of a particular
liquid/solid combination. After all, if you know the surface energy
of the solid, the surface tension of the liquid, then you should be
able plug those values into Young's equation and computationally
derive the contact angle. This overly simplified approach fails
to account for the impact of surface roughness on wetting, which can
be significant, and the delicate state of surface energy which can
dance around unpredictably as a result of contaminants, surface
treatments, environmental conditions, nanoscopic structures, and
chemical and structural homogeneity...or lack thereof. In short, contact
angle using the sessile drop technique remains the single most common
method for determining wettability in a complex system driven by a
variety of variables. |
| What Makes Model 210 Such a Great Instrument |
|
Everyone has favorites. I'm partial to
our Model
210 Goniometer / Tensiometer. It's a love affair that began the
day we introduced this model three years ago. Model 210 uses the
same optical bench, backlighting, and camera that all of our
higher-end instruments use. In fact, the hardware is identical to
Model 250.
However, the new
DROPimage Pro software that we also released in 2018 makes Model
210 easy to use and yet powerful. Unlike the retired Model 200 which
measured contact angle and surface energy, Model 210 adds surface
and interfacial tension capabilities.
What makes this such a great starting tool is its capacity to be upgraded. Down the road, as your needs become more demanding, the software can easily be upgraded to DROPimage Advanced - adding a methods-based experiment design tool that is ideal for dynamic and time-dependent studies. The Automated Dispensing System can also easily be added to increase drop volume precision and speed up drop dispensing and accuracy. The Automated Dispensing System is also ideal for doing advancing and receding measurements using the add / remove volume method. Many other accessories are also available and can easily be added: The Environmental Fixture for doing captive bubble and liquid/liquid studies, the Hot Plate or Heated Environmental Cell for doing temperature-controlled studies, the Automated Tilting Base for doing advancing and receding studies using the tilting plate method. In short, there are over two dozen accessories available for Model 210 that can provide the necessary platform for a powerful array of surface analysis studies. |
|
Regards,
Carl Clegg |